Science
Human milk contains a myriad of bioactive carbohydrate-containing molecules (glycans) that are now widely recognized to provide a number of important health benefits. In recent years the pace of research has accelerated on the most abundant class of human milk glycans, the human milk oligosaccharides (hMOS).
Specific hMOS have been found to be anti-infective, anti-inflammatory, prebiotic, and/or to promote mucosal development and immunity. Studies conducted by Glycosyn’s founding scientists and others have identified individual hMOS with great translational potential as novel agents to promote health, healthy developments, and to reduce risk of infection. Human Milk Oligosaccharides (hMOS) are currently thought to provide health benefits in three main areas:
- Establishment and maintenance of a healthy gut microbiota. An increasing body of research has demonstrated that diet profoundly impacts the microbial composition of the gut. hMOS, which are absorbed poorly by the infant intestine, remain available in the gut lumen where they promote the establishment of a beneficial gut microbiota. Important bacterial commensal species, e.g. members of the Bifidobacteria and Bacteroides genera, can efficiently utilize the hMOS of mother’s milk as a carbon source and thus gain a selective advantage for gut colonization, while the growth of undesirable bacteria that cannot utilize hMOS, such as pathogenic Clostridia species, is suppressed.
- Immune function. Changes in the microbial composition of the gut caused by the presence of hMOS may indirectly influence immune function in infants. In addition, an increasing number of studies have demonstrated direct impacts by individual hMOS on the immune system. For example; sialylated hMOS have been shown to promote cytokine production from T-cells in vitro, sialyl-Lewis X (a structure mimicked by the hMOS “3’-S3FL”) can inhibit leukocyte rolling and adhesion (an early step in inflammatory processes), and the hMOS “LNnT” can reduce white blood cell production of the inflammatory cytokine IP-10 and promote the white blood cell production of the anti-inflammatory cytokine IL-10. Galactosyllactose and 2’-fucosyllactose also directly quench inflammatory signaling pathways.
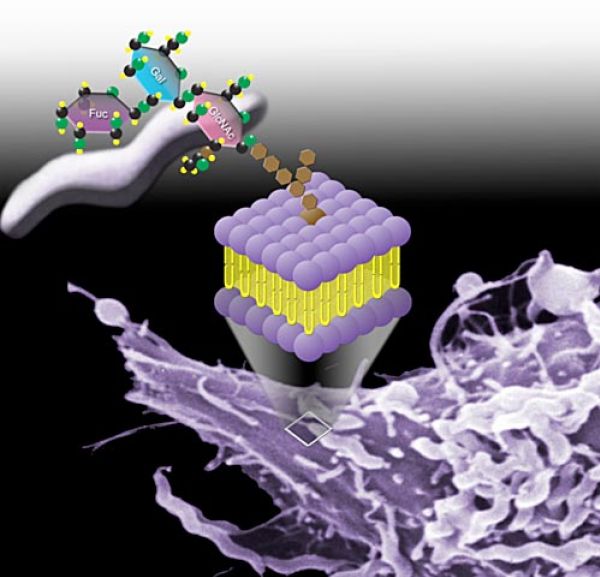
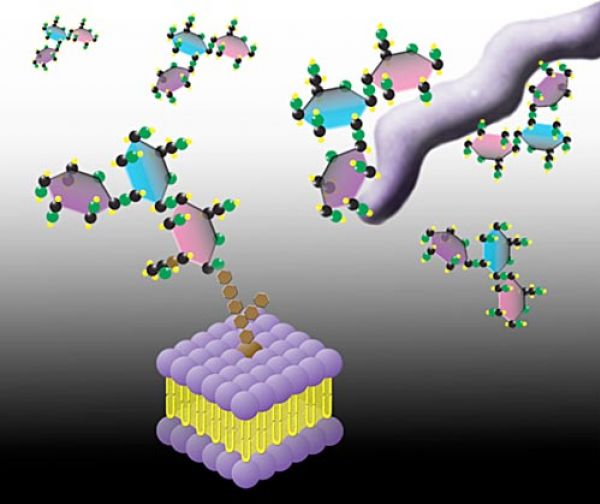
- Prevention of pathogen adhesion and infection. Adherence of pathogens to host cells is the obligatory first step in infection and it is frequently mediated by specific molecular interactions. Several leading bacterial and viral causes of human infectious diarrhea adhere to gut epithelial surfaces through binding to specific sugars (i.e. α(1,2) fucosylated glycans) expressed on the surface of intestinal cells. Other pathogens, including both gut and respiratory pathogens, are known to bind to their hosts through sialic acid-containing glycans found on the surface of their particular host target cell. These same α(1,2) fucosylated and sialylated glycans are also found in soluble forms in the hMOS fraction of human milk. Soluble α(1,2) fucosylated glycans present in human breast milk competitively inhibit the adherence of several major pathogens to gut epithelia, including strains of Campylobacter jejuni, Vibrio cholerea, Escherichia coli, Salmonella enterica, and Noroviruses. hMOS thus represent a new class of natural anti-infective agent that provides an important layer of innate prophylactic protection to young infants.
For most nursing children, breastfeeding is the best path to healthy growth and development. It remains the best way to reduce or eliminate the risk of diarrhea from many common pathogens. However, nursing children whose mothers cannot provide sufficient protective α(1,2) fucosylated glycans in their milk due to genetic makeup (over 20% or more of most populations), and many more millions of weaned and prematurely weaned children (particularly in poor populations), are currently at serious risk of disease and death from infectious diarrhea. Glycosyn’s anti-adherence anti-infectives merely make the pathogen’s environmental niche unavailable, and thus will not result in antibiotic resistance. Even after millennia of opportunity, pathogens have not evolved mechanisms to circumvent the natural anti-infective glycans present in mother’s milk.